Effective Concentration And Conditions of Ozone Disinfection!
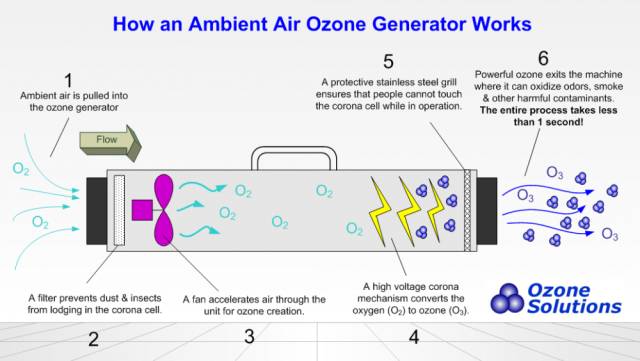
Although many companies in China use ozone to disinfect clean rooms, many people in the industry have doubts about its disinfection effect. This article summarizes the requirements of different domestic and foreign laws/guidelines on the concentration and conditions of ozone disinfection for your reference:
GB 28232-2020 "Sanitary Requirements for Ozone Sterilizers":
Air disinfection ozone concentration: 5 mg/m3~30 mg/m3, relative humidity ≥70%, the effect will be 30~120min.
Surface disinfection: ozone concentration should be ≥60 mg/m3, relative humidity ≥70%, and the action time should be 60 min~120 min.
When using ozone water to disinfect the surface of an object: the ozone concentration in the water should be ≥10 mg/L, and the action time should be ≥60 min.
The disinfection effect should meet the following standards:
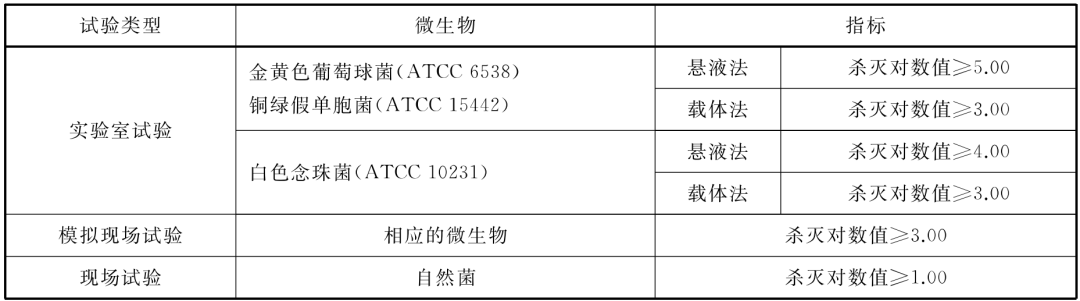
Air disinfection
• When the ozone sterilizer is used for air disinfection, it should meet the following requirements:
Killing microbe indicators when disinfecting air
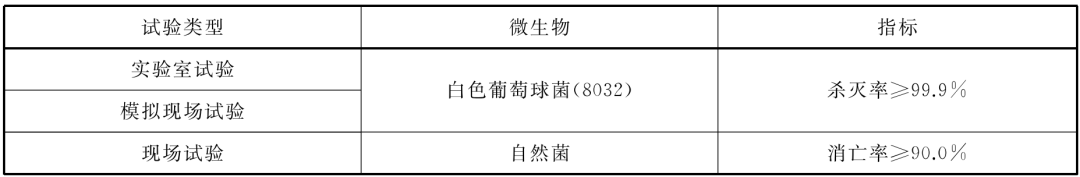
Water disinfection
• When the ozone sterilizer is used for water disinfection, it should meet the following standards:
Index of killing microorganisms when disinfecting water
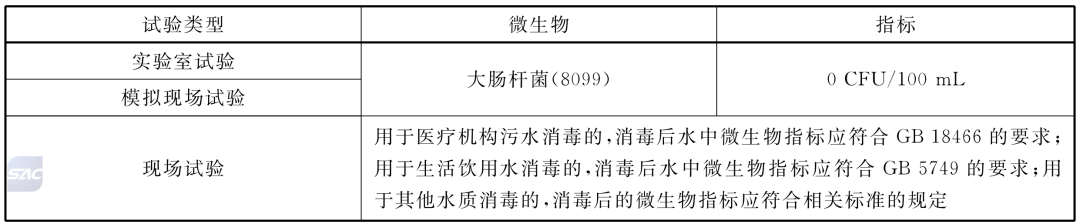
Disinfection of medical equipment and supplies
• When the ozone sterilizer is used to sterilize medical equipment and supplies, it should meet the following standards:
Killing microbes when disinfecting medical equipment and supplies
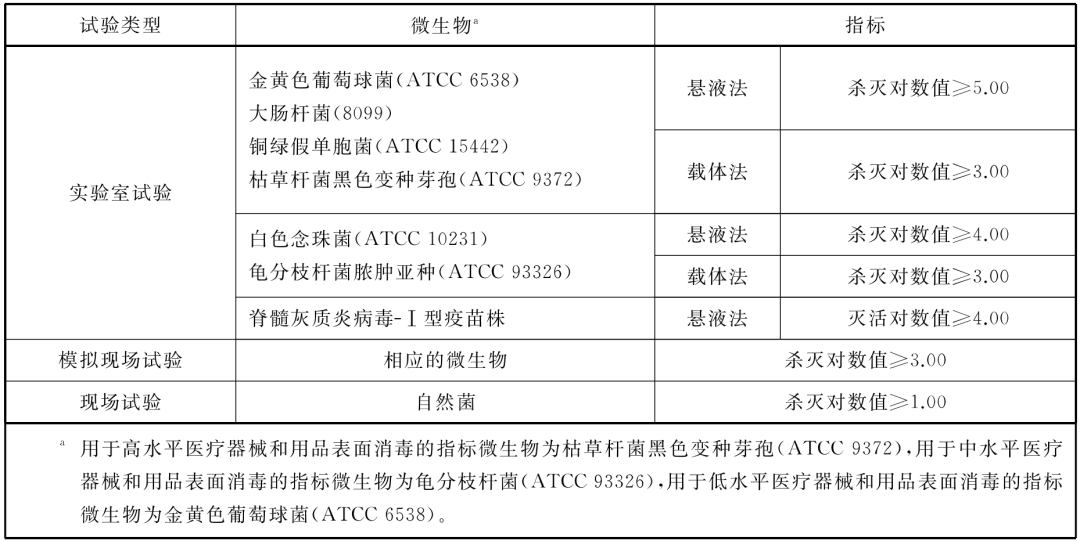
Surface disinfection
• When the ozone sterilizer is used to sterilize objects, it should meet the following standards:
Index of killing microorganisms when disinfecting the surface of objects
Verification Guide
When disinfecting, close the corresponding fresh air inlet and return air discharge valve, so that the entire disinfected clean area air will circulate through the air duct of the purification system, and the ozone generator will start to work. If air sterilization is performed daily, it can generally be turned on for 1 to 1.5 hours; if ozone is used to replace chemical reagent fumigation to sterilize surfaces, walls, floors and equipment every week, it can generally be turned on for 2 to 2.5 hours.
For airborne bacteria, the concentration of ozone sterilization is (2~4)×10^-6 (2~4ppm); for sedimentation bacteria on the surface of the object, it is (10~15)×10^-6 (10-15ppm)
After designing and applying ozone sterilization for 60 minutes to reach a relative concentration, continue to maintain for a period of time (1~1.5h) to achieve the purpose of killing bacteria settled on the surface of machinery, equipment and buildings.
Principles of cleaning and disinfection procedures for PDA TR 70 aseptic production facilities
The use of Ozone Gas is another alternative for gassing small or large scale operations. Ozone is made by adding high voltage to oxygen. The system uses a high concentration of ozone gas that bintegrates a gas generator to emit the Ozone to the area to be decontaminated. Normally the design specifications for the system included an ozone gas concentration of 200 ppm or more, relative humidity of 80% or more, and a treatment time that is determined by the size of the area, the inherent bioburden and the obstructions contained within the area. These systems have been employed in many industry settings and are now being considered as a possible alternative for GMP operations.
Whenever chemical agents are used for large-scale gassing or fogging of clean rooms, safety concerns must be addressed. All of the agents discussed can result in injury or death of personnel if proper precautions are not taken to ensure the containment of the chemical agent to the intended areas.
For many of the agents discussed, residues that are left behind on product-contact surfaces are also a significant concern and must be evaluated.
Although these methods of decontamination are effective, they should not be used to replace a routine program for cleaning and disinfecting the clean room areas. If they are used as the standard practice, they should be validated to demonstrate their ability to achieve an appropriate level of bioburden reduction. This should be performed taking into consideration the material of construction present in the clean room areas.
It is also important to consider the source of these organisms, for gassing will only remove what is present and may leave behind moisture, allowing for further proliferation if the causative agents have not been removed from the area.
References
The ozone gas concentration is 200 ppm or higher, the relative humidity is 80% or higher, and the sterilization time is 120 minutes. By placing a ventilation device to ensure uniform air flow, the air flow can cover the entire room during the ozone gas sterilization process. The concentration target value obtained by multiplying the ozone gas concentration during the ozone gas sterilization process by the sterilization time (mg / m(3) (= ppm) × min) is equal to or greater than 24 × 10 (3) (ppm·min) ). A biological indicator (BI) was used to confirm the disinfection results in the clean room, and negative results were obtained at all measurement points, which indicated that disinfection was effectively performed in the actual factory where the ozone gas disinfection system was introduced.
——Biocontrol Sci. 2013;18(1):9-20. doi: 10.4265/bio.18.9.Confirmation of the sterilization effect using a high concentration of ozone gas for the bio-clean room,Takuji Iwamura 1, Katsunori Nagano, Toshihiro Nogami, Noritomo Matsuki, Noriyoshi Kosaka, Hideharu Shintani, Miyoshi Katoh
Residual concentration after ozone disinfection
GB/T18883 "Indoor Air Quality Standard": 0.16mg/m3